Hot acid leaching of a large amount of iron and slag jarosite leached high alumina zinc refining process method, slag and high jarosite leached zinc oxide rotary kiln dust reduction after evaporation. The soot is rich in more than 90% of indium and more than 95% of zinc in the slag and high-dip slag, and is also enriched with impurities such as arsenic and antimony . The soot contains 2% of indium, 55% of zinc, and 3% of arsenic. , 锑 2%. In annual electricity meter scale zinc 55,000 t, jarosite yield the slag 44 000 t, t 11000 high leaching residue, reducing volatile fumes can produce 6885t, China Tin Group to the guest volatile reduction smelter soot pilot indium containing 1.99% Zinc is 54.97%, lead is 2.56 percent, and tin is 4.33%. It contains 138t of indium, 3785t of zinc, 176t of lead, and 298t of tin. Such as zinc, indium and other valuable metals recovery well, with great economic benefits. In this paper, in order to reduce the characteristics of volatile zinc oxide soot, a new process of sulphuric acid ripening and leaching is adopted to improve the leaching rate of zinc and indium of soot. The extraction of indium and the raffinate are used to remove impurities, thereby effectively recovering zinc and indium. In addition to the purpose of impurities such as arsenic and antimony.
1. Test materials and methods
(1) Test materials
The test zinc oxide soot is provided by the Huaxi Group Laibin Smelter, which is produced by the semi-industrial test of the zinc system open-circuit slag rotary kiln. Its chemical composition is shown in Table 1.
Table 1 Chemical constituents (mass fraction) of zinc oxide soot
Zn | In | Fe | As | Sb | Pb | Sn | Si | C | S |
45.87 | 1.91 | 3.64 | 3.49 | 1.94 | 3.64 | 3.67 | 0.90 | 1.30 | 3.20 |
It can be seen from Table 1 that the composition of the zinc oxide soot is complicated, and the content of zinc and indium is lower than that of the produced soot, and the content of impurities such as iron and arsenic is high.
(2) Test instruments and research methods
The leaching section and the impurity removal test were carried out in a 1.0 L glass beaker, a certain volume of the reaction slurry was added, and the stirrer was started at the same time to adjust the stirring strength to fully disperse the slurry, and the reaction temperature was controlled by a Beckman thermometer and an electronic relay. And pH precision test paper to determine the pH value of the reaction process, vacuum leaching leaching slag for solid-liquid separation, taking the solution and slag for chemical analysis. The low supernatant was sent to a 0.5 and 1.0 L separatory funnel for extraction and indium extraction.
Second, the process and process conditions
(1) Process
According to the nature of the test zinc oxide soot raw materials, the design principle flow is shown in Figure 1. The process consists of four main processes: concentrated acid ripening, three-stage leaching, extraction and indium addition, neutralization and impurity removal. The open slag is washed in three stages of countercurrent, and the washing liquid is returned to the system. The leaching slag is rich in lead and tin, and can be used as a raw material for recovering lead and tin. A small amount of indium is recovered in the production of lead and tin, and can also be returned to the reduction kiln for reprocessing. The metal (including impurities) which is matured by concentrated acid and leached by water is collected in a low acid leaching solution. After indium extraction, the impurities are removed by neutralization and removal of impurities such as iron, arsenic and antimony. Leaching. The open-dip immersion supernatant is supplied to the zinc purification process or purified and concentrated to prepare a zinc sulphate product. The full process solution constitutes a closed loop.

Figure 1 Flow chart for reducing the amount of zinc and indium from the volatile fumes
(If the picture is unclear, you can call it for free)
(2) Process conditions
1. Medium immersion: the initial acid is 49.45g/L, the end point pH is 5.0-5.2, the reaction temperature is 55-65°C, and the reaction time is 60min (according to the control end pH value).
2. Low immersion: the initial acid is 39.67g/L, the final acid is 10g/L, the reaction temperature is 75°C, and the reaction time is 60min.
3. Concentrated acid ripening: low slag amount / concentrated sulfuric acid amount is 1/0.98, curing temperature is 85 ° C, and curing time is 3 h.
4. Water-soluble leaching: the solid-liquid ratio is 1/5, the initial acid is 120-130 g/L, the final acid is 119.2 g/L, the reaction temperature is 70-90 ° C, and the reaction time is 1 h.
5, extraction: extract concentration of 30% P 204 + coal oil (volume ratio), O / A = 1/ 2, the extraction temperature is room temperature, the extraction time is 2 ~ 4min.
6, impurity removal: the feed rate is 450mL / h, the reaction pH is controlled to 4.0 ~ 5.0, the reaction temperature is 90 ° C, the reaction time is 150min.
Third, the test results
The experiment was carried out for 23 cycles. The first 13 cycles were the liquid-forming and adjustment phases, and the 14-23 cycles were the test data. The test comprehensively measured various technical indicators, changes in ion concentration of each process, and solution and slag. Physical and chemical parameters, etc.
(1) The average composition of each process solution and slag
The average composition of each process solution and slag is shown in Table 2 and Table 3.
Table 2 Average composition of each process solution / (g · L - 1 )
Solution name | Zn | Fe | In | As | Sb |
Medium immersion liquid | 129.93 | 0.003 | 0.005 | 0.016 | 0.006 |
Low immersion liquid | 109.82 | 3.52 | 2.75 | 0.949 | 0.169 |
Aqueous liquid | 42.98 | 13.15 | 4.47 | 0.47 | 0.33 |
Raffinate | 108.42 | 2.57 | 0.01 | 0.949 | 0.169 |
Dust removal | 100.16 | 0.20 | - | 0.010 | 0.014 |
Table 3 Average composition (mass fraction) /% of each process slag
Slag name | Zn | Fe | In | As | Sb | Zn water soluble | Pb | Sn |
Medium slag | 28.92 | 4.8 | 2.35 | 4.38 | 2.34 | 8.66 | - | - |
Low slag | 13.16 | 5.03 | 2.62 | 6.26 | 3.61 | - | - | - |
Leaching residue | 0.68 | 5.68 | 0.37 | 11.17 | 7.14 | - | 14.16 | 13.67 |
Deodorization | 0.154 | 4.33 | 0.012 | 1.91 | 0.35 | - | 0.006 | 0.007 |
From the average composition of the solutions listed in Table 2, the solution components of each process are basically consistent with the process design requirements. The medium immersion supernatant requires zinc containing 130g/L, and the low supernatant contains acid 10-15g/L. The test results showed that the upper supernatant contained 129.93 g/L of zinc and the low supernatant contained 11.59 g/L of acid, all within the required range. In addition, it can be seen from the average composition of each process solution that after the raffinate is removed, the iron removal rate in the solution is 85.01%, the arsenic removal rate is 95.22%, and the cockroach removal rate is 94.92%.
From the average composition of the slags listed in Table 3, it is known that the leaching slag and the removing slag contain very low zinc. The leaching slag contains lead and tin, and more than 90% of the lead and tin in the soot are enriched in the leaching slag, which is beneficial for further recovery of lead and tin. The indium is recovered in the production of lead and tin, and can also be returned to the reduction kiln. Reprocessing.
(2) slag volume and slag rate
The slag amount and slag rate of various slags are shown in Table 4. The intermediate slag rate is calculated by sampling the measured moisture. In this experiment, 3 # anionic flocculant was added in neutral leaching, 3 # cationic flocculant was added in low acid leaching and water leaching, and 2 kinds of flocculants were added to neutralize and remove impurities. The clarification effect was good, the supernatant was clear, and the supernatant was clear. The rate is greater than 60% (60 min). Industrial production is carried out in a dense and continuous operation, and the clarification effect is expected to be better.
Table 4 slag amount and slag rate
Medium leaching residue | Low slag | Leaching residue | Deodorization |
Slag amount / g | Slag rate /% | Slag amount / g | Slag rate /% | Slag amount / g | Slag rate /% | Slag amount / g | Slag rate /% |
102.45 | 71.64 | 68.78 | 48.10 | 35.62 | 24.91 | 41.71 | 29.17 |
(3) Leaching rate of metal and the destination of impurities
According to the amount of the leaching slag and the element content, the metal leaching rate and the impurity slag rate are calculated. The total leaching rate (%) was: zinc 99.63; indium 95.13; iron 61.11; arsenic 20.48; 锑8.37; lead 4.32; tin 7.64. The enrichment slag rates (%) of arsenic, antimony, lead and tin were 79.50, 91.63, 96.55 and 92.37, respectively. Arsenic and antimony entering the leachate were 95.22% arsenic and 94.92% in the decontamination process. This product has a good ability to remove impurities such as arsenic and antimony.
(four) extraction
P 204 using indium extraction, the extraction yield as high as 99.63 percent indium, about 1.47% iron. It shows that P 204 has strong selective extraction ability for indium. If the content of impurities in the extract is high, the extraction is easy to emulsify, and after adding a certain amount of polyether, the phenomenon of extraction and emulsification can be eliminated.
Fourth, discussion
(1) Process analysis
In view of the general high acid leaching method for reducing volatile fumes, indium is difficult to leaching and the leaching rate is low. This process uses concentrated acid leaching for the first time, which enhances the leaching method and leaching conditions, and greatly improves the leaching rate of indium. According to the difference in the extraction speed of indium and iron, P 204 is directly extracted from the low acid leaching solution, and the acidity should be controlled at 10-15 g/L. The process achieves the purpose of "zinc, indium, and comprehensive utilization". Zinc is opened from the neutral leachate; indium is recovered from the low acid leach solution; lead and tin are concentrated in the leach residue for easy recovery.
(2) Removal of impurities
The concentrated acid ripening enhances the leaching process, so that more than 99% of the zinc and more than 95% of the indium enters the solution, and at the same time, many impurities are also leached into the solution. In the process of neutralization and impurity removal, the impurities are well removed, and the removal rate (%) is: arsenic 95.22, 锑94.92, iron 85.01, and Ï(Fe 3+ )<1.0g/L after impurity removal, arsenic and antimony Around 0.01 ,, the quality of the immersion liquid in the return is guaranteed. It is recommended to return white arsenic in addition to the slag to recover the arsenic.
(3) Metal recycling
From soot to neutral leachate, the recovery rate of zinc smelting is 99.56%. The neutral leachate composition (g/L) is: zinc 129.93, iron 0.0025, arsenic 0.016, 锑 0.0053, which can be used to supply electricity to the zinc purification process or to produce other products.
The trend of indium in each process was investigated. The average indium leaching solution of low acid leaching solution was 2.75g/L. According to the indium containing leaching slag, the indium leaching rate was 95.13%. According to the average content of indium in low immersion liquid, the indium leaching rate was 94.18. %, the indium was extracted directly from the low acid leaching solution, and the indium extraction rate was 99.63%.
V. Conclusion
The new process of immersion in concentrated acid ripening water is used to enhance the leaching process conditions under the condition of constant acid dosage. The leaching rate of indium and zinc is high, and the leaching rate (%) of the slag meter is: indium 95.13, zinc 99.63; arsenic and antimony The lead and tin slag rates (%) were 79.51, 91.63, 96.55, and 92.37, respectively. In the extraction process, a small amount of polyether is added to prevent emulsification. Indium was extracted directly from the low acid leaching solution with P 204 , and the first extraction rate of indium was as high as 99.63%. The raffinate was neutralized with CaCO 3 to remove impurities. At the same time as the iron ore was removed, 95.22% of the arsenic in the raffinate and 94.92% of the antimony entered the iron slag. After the decontamination, the liquid contained 0.20 g/L of iron. Arsenic 0.010 g / L, 锑 0.014 g / L. The whole process is smooth and stable, and the purpose of effectively recovering zinc, indium and effectively removing arsenic and antimony is achieved. For the better process of treating similar soot, it can be considered for production practice.
Wedge wire screen filer elements are made of stainless steel wedge wire screen. Filter rating is from 30 to 80 microns. Wedge wire screen is welded onto rods with stainless steel wedge wire at every contact point. They are classified into filer sheet, filter basket and filter elements. They have such characteristics as good strength, high ridity, resistance to abrasion and corosion, even gap, good in filtration and fluidity, easy to clean and back wash.
Application Wedge wire screen filter elements are used in sieving of petroleum, chemical industy, pharmaceutics, food and beverage, metalurgy and coal, also can be used in fitration of water treatment.
Wedge wire is a welded steel structure, mainly used for filtration, separation and retention media.
It consists of surface profiles, usually V-shaped, that are resistance welded onto support profiles. The distance between the surface profiles is controlled very accurately, as it forms the slot through which the filtrate flows.

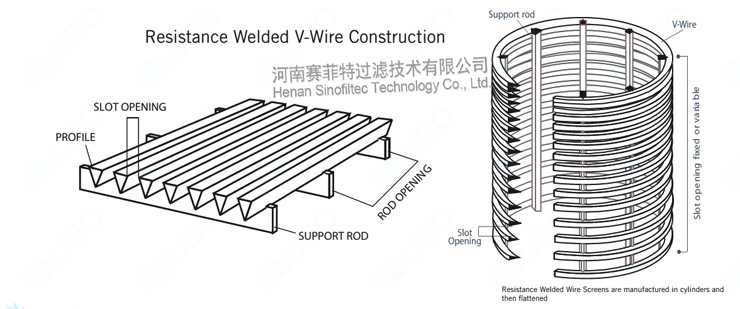
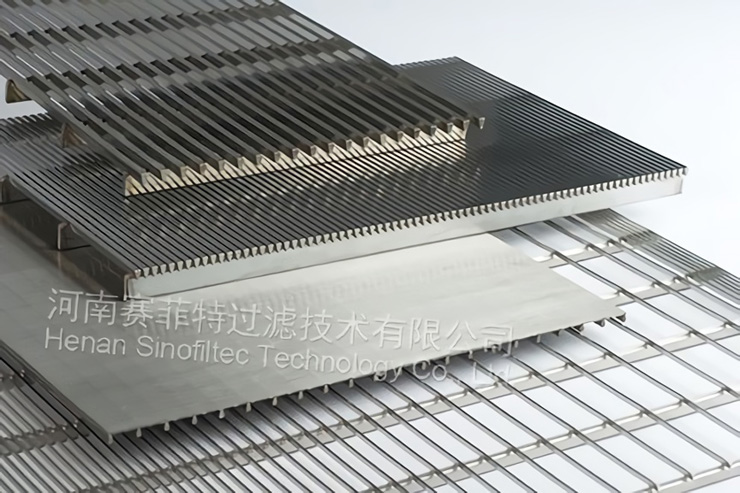
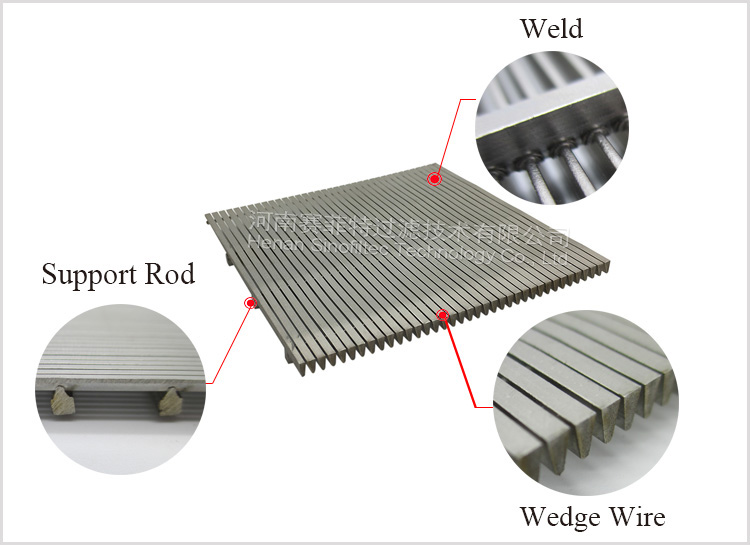
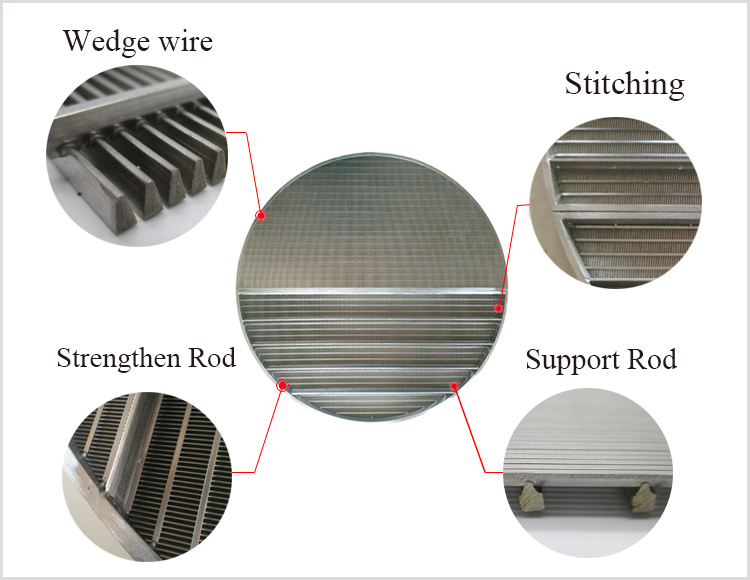
Wedge Wire Screen Filters
Stainless Steel Filter Cartridge,Wedge Screen Filter Cartridges,Wedge Wire Screen Filters
Henan Sinofiltec Technology Co.,Ltd , https://www.sinofiltec.com






![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)