[ Instrument Network Instrument R & D ] Recently, Huang Xingjiu, a researcher at the Institute of Intelligent Machinery, Hefei Institute of Material Science, Chinese Academy of Sciences, and Zeng Jie, a professor at the University of Science and Technology of China, used the Co monoatomic catalyst (Co) dispersed on nitrogen-doped porous carbon for the first time. SAC) enables ultra-high sensitivity and selective electrochemical detection of As (III). At the same time, combined with synchrotron radiation X-ray absorption fine structure (XAFS) technology, density functional theory calculation (DFT), and kinetic simulation calculation, the interaction between Co SAC and H3AsO3 in the electrochemical analysis process was explored in detail from the perspective of electrocatalysis. Mode of action and working mechanism.
Electrocatalysis is a catalytic action that accelerates the charge transfer reaction at the electrode and electrolyte interfaces. The scope of electrode catalysts is limited to electrical materials such as metals and semiconductors. Electrocatalysis has been studied more on framework nickel, nickel boride, tungsten carbide, sodium tungsten bronze, spinel and tungsten ore semiconductor oxides, and various metal compounds and phthalocyanine catalysts. It is mainly used in the electrocatalytic treatment of organic sewage; the electrocatalytic degradation of chromium-containing wastewater; the electrolytic desulfurization of flue gas and raw coal; the electrocatalytic removal of NOx and S02 simultaneously; the electrolytic reduction of carbon dioxide and nitrogen.
In recent years, various nanomaterials such as precious metals, alloys, carbon materials, metal oxides and their composites have been widely used in the electrochemical analysis of As (III). However, most studies mainly rely on empirical tests, focusing only on electrochemical phenomena such as detection sensitivity, detection limit, and selectivity, and ignoring the corresponding detection mechanism. Therefore, we want to truly achieve high sensitivity and selection of As (III) in water. Sex testing remains a huge challenge. In fact, the essence of electrochemical detection is the process of electrocatalytic reaction at the sensing interface. This reaction will affect the enrichment / reduction of As (III) and the corresponding dissolution / oxidation signal. However, there is still a lack of science from the perspective of electrocatalysis. understanding. Therefore, exploring the interaction between the sensing interface and the target analyte, and clarifying the corresponding electrocatalytic mechanism, is very important to develop an effective functional sensing interface to achieve high sensitivity and selective detection of As (III).
Electrocatalysis covers two aspects of electrode reaction and catalysis, so electrocatalyst must have these two functions at the same time: â‘ can conduct electricity and transfer electrons relatively freely; â‘¡ can perform effective catalytic activation of the substrate. Not all electrically conductive materials have an activating effect on the substrate and vice versa. Therefore, a feasible way to design an electrocatalyst is to modify the electrode. By combining the active component with a form of covalent bond or chemisorption on a substrate electrode that can conduct electricity, the dual purpose of transferring electrons and activating the substrate can be achieved. Of course, in addition to considering the macroscopic mass transfer of the electrode, there is also a problem of the interaction between the modified molecule and the substrate electrode, and this interaction needs further study.
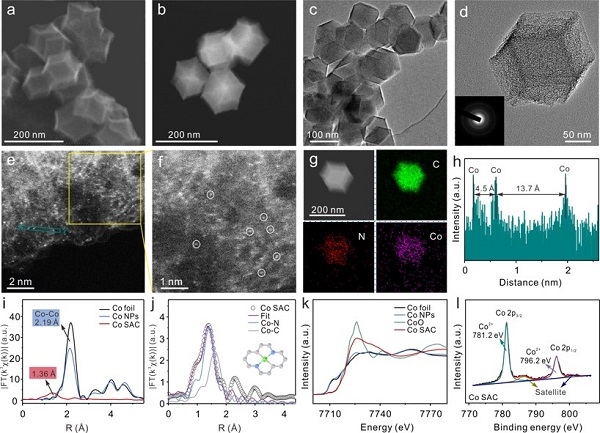
Based on the above problems, the researchers successfully synthesized a Co SAC catalyst and used it for the electrochemical detection of As (III), which can achieve ultra-high sensitivity of 11.44 μA ppb-1 and excellent selectivity for As (III). This sensitivity is high among currently reported non-precious metal catalysts, and is even better than most precious metal nanomaterials. Combining XAFS and DFT calculation results, the researchers found that H3AsO3 molecules were catalytically activated by Co-N2C2 active sites and formed Co-O hybrid bonds during the reaction, which resulted in a reduction in the energy barrier of the As-O dissociation step during the reduction of H3AsO3. In addition, simulation calculations of reaction kinetics show that the first electron transfer process is the rate-limiting step for the entire reduction of H3AsO3, which is much faster on Co SAC than on Co nanoparticle materials (Co NPs), thereby promoting the As (0) The rapid and massive deposition greatly enhanced the electrochemical response signal of As (III). The selectivity for As (III) is also attributed to the formation of Co-O hybrid bonds, because there is no specific interaction site between Co SAC and ordinary divalent heavy metal ions that do not contain oxygen anions.
The original meaning of heavy metals refers to metals with a density greater than 4.5g / cm3, including gold, silver, copper, iron, mercury, lead, and cadmium. The accumulation of heavy metals in the human body reaches a certain level, which will cause chronic poisoning. However, in terms of environmental pollution, heavy metals mainly refer to heavy elements with significant biological toxicity such as mercury (mercury), cadmium, lead, chromium, and metal arsenic. Heavy metals are very difficult to be biodegraded. On the contrary, they can be enriched hundreds of times under the action of biological amplification of the food chain, and finally enter the human body. Heavy metals can strongly interact with proteins and enzymes in the human body, causing them to lose their activity, and they may also accumulate in some organs of the human body, causing chronic poisoning.
This work is the first use of a single-atom catalyst to construct a sensing interface for the electrochemical analysis of heavy metal ions, expanding the application of single-atom catalysts in the field of electrical analysis. Through advanced XAFS characterization, DFT calculations and dynamic numerical simulations, atomic and electronic structures can be effectively linked to macro-electrochemical phenomena, indicating that the electrocatalytic ability of sensing materials is sensitive and selective in the detection of analytes. It plays a vital role. Research on the working mechanism of Co SAC provides atomic-level catalytic insights and important theoretical guidance for designing functional sensing interfaces in environmental applications. At the same time, it also provides inspiration for catalytic treatment and removal of such pollutants in the water environment.
Source: Hefei Institute of Material Science, Encyclopedia
Jiangmen Yingzhihui Electronic Commerce Co., LTD , https://www.yzhprint.com
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)